Classification of Lipids
Although the term “fat” is used for all fat and similar molecules in the colloquial language, fats and oils are molecules in the group called “lipids” in food chemistry. However, there is no standard view on classifying lipids in the scientific community. In general, lipids are divided into three classes.
1) Simple Lipids
a) Fats and Oils; All of the fats and oils used as edibles are in this class. Fats are molecules formed due to the ester bond between a glycerin molecule and three fatty acids. More detailed information is given below.
b) Waxes; are lipids formed due to the combination of long-chain fatty acids and alcohols.
c) Color waxes; are lipids formed by combining fatty acids with double bonds and alcohols with double bonds.
d) Sterol esters; are lipids formed by the combination of fatty acids and sterols. Cholesterol is an example of such a lipid.
e) Triterpenic alcohol esters; are lipids formed by combining triterpenic alcohols and fatty acids.
2) Compound Lipids
a) Phosphorus and nitrogen-containing lipids; Lecithin, cephalin, acetal phosphatides and sphingomyelins are examples of this group of lipids.
b) Carbohydrate-containing lipids; Cerebroglycosides, cerebrogalactosides, gangliosides and sulfatides are examples of this group of lipids.
c) Protein-containing lipids; They are lipoproteins formed due to the combination of lipids and proteins of different structures.
3) Derived Lipids
Fatty acids, hydrocarbons, fat-soluble vitamins and colorants, antioxidants, higher alcohols, flavor and odor substances are classified as derived lipids.
Fats and Oils (Triglycerides)
Fat molecules are made up of carbon (C), hydrogen (H) and oxygen (O). They are insoluble in water and dissolve in most organic solvents.
Fats are formed by the combination of glycerin, which trivalent alcohol and fatty acids; They are organic molecules called “triglycerides.” “Simple triglyceride” is if the fat molecule contains only one type of fatty acid; If it has two or three different fatty acids, it is called “mixed triglyceride.”

Molecules formed by attaching only one fatty acid to glycerin are called “monoglycerides”; The molecules formed when two fatty acids are bonded are called “diglycerides.”
While the foods we consume as fat contain about 98% triglycerides; It includes 1-1.5% mono and diglycerides. The digestion of mono and diglycerides in the body is not different from triglycerides. However, mono and diglycerides show emulsifying properties. Due to these properties, they are food additives used extensively in the food industry to create emulsions.
Triglycerides can be found in solid or liquid form at room temperature. The fatty acid profile determines whether triglyceride is in solid or liquid form and its hardness in the solid state. While a triglyceride’s high content of saturated fatty acids causes it to be solid at room temperature; Its high unsaturated fatty acid content allows it to be in liquid form at room temperature.
In addition, while the amount of short-chain fatty acids is high, the melting point is lower; The higher the amount of long-chain fatty acids, the higher the melting point.
If we evaluate the example of butter; The high amount of saturated fatty acids it contains ensures that butter is solid at room temperature. However, the fatty acid profile of butter is variable.
Generally, butter’s unsaturated fatty acid content increases in spring and summer. This makes the butter produced in the spring and summer months softer and spreadable. At the same time, the decrease in the amount of unsaturated fatty acids in the winter and autumn seasons causes the butter to have a more rigid structure.
The physical, chemical and physiological properties of triglycerides are determined by the “fatty acids”, the building blocks they contain.
Fatty acids
Fatty acids are organic molecules, usually straight-chain, containing an alkyl group and a carboxyl group (-COOH). Over 500 different fatty acids have been identified in nature and it’s known that they include at least two and at most 26 carbons. However, fatty acids containing between 4 and 22 carbons are found in foods and are important in food chemistry.
Milk fat and butter are the wealthiest fats in fatty acid diversity. So far, over 400 different fatty acids have been identified in milk fat. However, while 15 of these fatty acids constitute 99% of the fatty acid presence, others are in trace amounts. Approximately 30 different fatty acids form 99% of the fatty acid profile in all fats and oils consumed as food.
These fatty acids are given in the table; (If you are viewing from a mobile phone, select the desktop site version to view correctly)
| Common name | Systematic name | Chemical structure | Melting point (oC) | Info |
| Butyric acid | Butanoic acid | C4H8O2 | -8,0 | Saturation; Saturated Rich Foods; Milk, Butter |
| Caproic acid | Hexanoic acid | C6H12O2 | -3,4 | Saturation; Saturated Rich Foods; Milk, Butter |
| Caprylic acid | Octanoic acid | C8H16O2 | 16,0 | Saturation; Saturated Rich Foods; Milk, Butter and Coconut oil |
| Capric acid | Decanoic acid | C10H20O2 | 31,3 | Saturation; Saturated Rich Foods; Milk, Butter, Coconut oil and Palm oil |
| Caproleic acid | Dec-9-enoic acid | C10H18O2 | – | Saturation; Monounsaturated, a double-bound Rich Foods; Milk, Butter |
| Lauric acid | Dodecanoic acid | C12H24O2 | 43,5 | Saturation; Saturated Rich Foods; Milk, Butter, Laurel oil, Coconut oil and Palm oil |
| Lauroleic acid | (Z)-dodec-9-enoic acid | C12H22O2 | – | Saturation; Monounsaturated, a double-bound Rich Foods; Milk, Butter |
| Myristic acid | Tetradecanoic acid | C14H28O2 | 54,4 | Saturation; Saturated Rich Foods; Most vegetable oils and animal fats |
| Myristoleic acid | (Z)-tetradec-9-enoic acid | C14H26O2 | -4,5 | Saturation; Monounsaturated, a double-bound Rich Foods; Milk, Butter, Fish oil |
| Palmitic acid | Hexadecanoic acid | C16H32O2 | 62,9 | Saturation; Saturated Rich Foods; Most vegetable oils and animal fats |
| Palmitoleic acid isomer cis7,cis9 | (Z)-hexadec-9-enoic acid, (Z)-hexadec-7-enoic acid, | C16H30O2 | 0-5 | Saturation; Monounsaturated, a double-bound Rich Foods; Fish oil, Milk and Butter |
| Margaric acid | Heptadecanoic acid | C17H34O2 | – | Saturation; Saturated Rich Foods; Milk, Butter and Meat |
| Stearic acid | Octadecanoic acid | C18H36O2 | 69,6 | Saturation; Saturated Rich Foods; Most vegetable oils and animal fats |
| Oleic acid | (Z)-octadec-9-enoic acid | C18H34O2 | 16,3 | Saturation; Monounsaturated, a double-bound Rich Foods; All vegetable oils and animal fats |
| Petroselaidic acid | (E)-octadec-6-enoic acid | C18H34O2 | 32,5 | Saturation; Monounsaturated, a double-bound Rich Foods; Parsley seeds |
| Vaccenic acid | (E)-octadec-11-enoic acid | C18H34O2 | 39,0 | Saturation; Monounsaturated, a double-bound Rich Foods; Animal fats |
| Linoleic acid | (9Z,12Z)-octadeca-9,12-dienoic acid | C18H32O2 | -5,2 | Essential fatty acid Saturation; Polyunsaturated, two double-bound Rich Foods; Milk, Butter and Vegetable oils |
| Conjugated linoleic acids (CLA) (9,11 and 10,12) | Cis9,tr11-CLA and tr10,cis12-CLA | C18H32O2 | – | Saturation; Polyunsaturated, two double-bounds Rich Foods; Milk, Butter, Cheese and Animal fats |
| Linolenic acid | (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid | C18H30O2 | -11 | Essential fatty acid Saturation; Polyunsaturated, three double bounds Rich Foods; Fish oil, Soybean and Rapeseed oil |
| γ- Linolenic acid | (6Z,9Z,12Z)-octadeca- 6,9,12-trienoic acid | C18H30O2 | – | Essential fatty acid Saturation; Polyunsaturated, three double bounds Rich Foods; Fish oil, Soybean and Rapeseed oil |
| Arachidic acid | Icosanoic acid | C20H40O2 | 75,4 | Saturation; Saturated Rich Foods; Peanut |
| Gadoleic acid | (Z)-icos-9-enoic acid | C20H38O2 | 23,5 | Saturation; Monounsaturated, a double-bound Rich Foods; Some fish oils |
| Arachidonic acid | (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid | C20H32O2 | -49,5 | Essential fatty acid Saturation; Polyunsaturated, four double-bounds Rich Foods; Pluck, Milk and Butter |
| Behenic acid | Docosanoic acid | C22H44O2 | 79,9 | Saturation; Saturated Rich Foods; Peanut and Rapeseed oil |
| Erucic acid | (Z)-docos-13-enoic acid | C22H42O2 | 33,5 | Saturation; Monounsaturated, a double-bound |
| Lignoceric acid | Tetracosanoic acid | C24H48O2 | 84,2 | Saturation; Saturated Rich Foods; Peanut and Rapeseed oil |
| Selacholeic acid | (15E)-tetracos-15-enoic acid | C24H46O2 | 39 | Saturation; Monounsaturated, a double-bound Rich Foods; Fish oil |
| Cerotic acid | Hexacosanoic acid | C26H52O2 | 87,7 | Saturation; Saturated Rich Foods; Trace in vegetable oils |
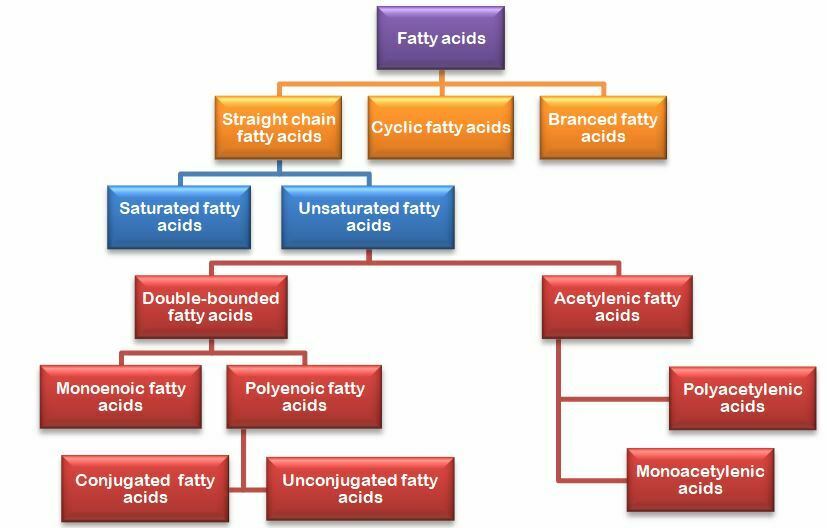
Classification of fatty acids
Fatty acids are divided into three classes; straight chain, branched and cyclic. However, the majority of fatty acids are in straight chain structure. As far as is known, branched and cyclic fatty acids are produced only by microorganisms and are included in the structure of the cell walls of microorganisms.
Branched and cyclic fatty acids secreted by microorganisms living in the digestive system of mammals can be found in the milk and in the butter produced from that milk of that animal.
These branched and cyclic fatty acids are passed in some tissues of people consuming milk and dairy products. In this way, some of the branched fatty acids are even used as indicators to determine whether humans consume dairy products.
Straight chain fatty acids are the most important class of fatty acids. The shortest chain fatty acid found in foods is the 4-carbon butyric acid. Fatty acids with 4-8 carbons are mostly found in milk and dairy products.
Straight chain fatty acids are divided into two classes as saturated and unsaturated fatty acids. Saturated fatty acids are fatty acids that do not have double or triple bonds in their structure. Unsaturated fatty acids have at least one double or triple bond in their structure.
Fatty acids are classified according to their molecular structures as follows;
Here’s an article that might interest you; Fats and Oils; Functions in the Body and Daily Needs