Milk is a food with a slightly acidic character. This acidic character is mainly due to the proteins (casein, albumin and globulins), phosphates, organic acids such as citric acid and carbon dioxide gas found in the natural structure of milk. This acidity is called “the natural acidity of milk.”
Since the natural acidity of milk depends on the components naturally present in its structure, any change in the amount or ratio of the components also causes a difference in the natural acidity of the milk.

For example, the natural acidity of colostrum, sheep, dog and whale milk, which has a higher protein content than cow’s milk, is also higher than cow’s milk. (For more detailed information about the composition of milk, see Milk; Definition, Composition and Nutrition)
However, the main factor that changes the acidity of milk is microorganism activities. Microorganisms “somehow” present in milk break down the lactose in milk and as a result of this breakdown, lactic acid is released. As lactic acid accumulates in the environment due to microorganism activity, the acidity of the milk increases. This is called “developing acidity”. (For detailed information about the microorganisms found in milk, see Microorganisms Found in Raw Milk; Types, Effects and Importance)
While there may be a limited change in pH value, such as 0.1-0.2, due to differences in the ratio of milk components, “developing acidity” due to microorganism activity can cause a significant change in the pH of milk, such as 3.0. The sum of the natural acidity of milk and the acidity that develops is expressed as “total acidity.”
What makes milk acidity very important is that the degree of acidity changes the structure, appearance and taste of milk. As a matter of fact, when the amount of acid in the milk reaches a certain level, the acidity in the environment causes the casein to coagulate (knowns as milk clotting or curdling of milk). Thus, milk moves away from its natural appearance as we know it and can take on different appearances, from slight particle formation to gelation, depending on the degree of coagulation. On the other hand, increased acidity causes milk to taste sour. (For detailed information about the sensory characteristics of milk, see Sensory Characteristics of Milk; Color, Taste and Appearance)
For the experts; The reason why casein coagulates is that the increase in acidity in milk causes the calcium in the structure of casein to pass into the serum phase. As calcium and phosphate ions separate from the casein micelle, the micelles weaken. The weakened micelles begin to break down, and obvious clots appear in the milk’s structure. At pH 5.5-5.8, approximately 50% of the calcium phosphate in the structure of the micelle passes into the serum phase. Demineralization is almost complete at pH 4.8-5.0. When the pH reaches 4.6, that is, the isoelectric point of casein, the total charge of the casein micelle is neutralized, and the micelle loses its colloidal structure irreversibly.
Milk is a food that cannot be consumed raw and must be subjected to a heat treatment such as pasteurization or boiling, whether consumed as drinking milk or processed into a product such as yogurt and cheese.
Milk, in its natural state, is a food that is highly resistant to heat. However, increased acidity in milk causes its structure to become unstable, and milk with high acidity becomes unable to maintain its structure when subjected to heat. When we boil the raw milk at home, we sometimes see that the milk curdles. The reason for this is the high acidity of raw milk.
Of course, this is related to the degree of acidity in the milk. Milk with high acidity levels will coagulate without being subjected to any heat treatment. Similarly, depending on the degree of acidity, different structural changes are observed under the influence of heat, from the observation of particles to the formation of curd.
As can be understood from this situation, increased acidity in raw milk is undesirable, and preventing and monitoring acidity is critical for the dairy industry.
While the development of acidity is generally undesirable in milk processing, the development of acidity is a desired and necessary condition in the production of yogurt and some types of cheese. For this purpose, the increase in acidity is generally encouraged by adding a starter culture after the milk is subjected to heat treatment and cooled.
The fermentation process when making yogurt at home consists of adding microorganisms to milk that increase acidity. During the fermentation process of milk, “lactic acid” is released due to the activities of microorganisms, and the acidity that develops causes the milk to gel; In other words, it enables it to turn into yogurt.
Apart from microorganism activity, if the acidity in newly milked milk is high, it may be suspected that the milk is colostrum or that some colostrum is mixed into the milk.
While the increase in acidity in milk is important for milk processing, milk acidity being lower than it should be is also an important indicator for different situations. It is suspected that preservatives have been added to milk whose acidity is lower than it should be or that the animal has mastitis (udder inflammation).
Milk acidity can be expressed in different units. These units are;
• pH,
• Soxhlet-Henkel (oSH),
• % Acidity (lactic acid),
• Dornic (oD) and,
• Thörner (oT).
The most common and widely used of these units is pH. Other commonly used units are Soxhlet-Henkel (oSH) and % acidity in terms of lactic acid (milk acid). Dornic (oD) and Thörner (oT) are known but not widely used units.
While pH is a standard unit for every substance to express acidity, the other four are milk-specific units used only in milk and dairy products.
Personally, in my nearly 15-year career, although I have used the determination method of the first three units thousands of times and although I have taught the Dornic and Thörner units in courses, I have never used the Dornic and Thörner units and never felt the need to use them.
Acidity is a concept that expresses the ion concentration of an environment. The most well-known acidity measurement method is pH, and while 7.0 is considered neutral, below 7.0 on a scale of 0-14 is considered acidic. We define values above 7.0 as basic. The point to note here is that pH decreases as acidity increases, and pH increases as acidity decreases.

The pH of milk freshly milked from a healthy animal and in its natural state is between 6.6 and 6.8 pH. The structure and appearance of milk are quite stable against heat at this acidity value.
As soon as the pH of raw milk drops below 6.4, milk curdling begins to occur as a result of heating. In particular, the pH of the milk to be used in the production of drinking milk and yogurt is not desired to be lower than 6.6. At pH values of 5.3-5.5, milk coagulates at room temperature without heating. If the pH is higher than 6.8, it is suspected that a preservative has been added to the milk or the milk has been milked from an animal with mastitis.
The pH value of milk varies depending on temperature. Every 10oC changes in temperature causes a 0.1 change in the pH level of milk. For example, the pH of milk, whose pH is 6.5 at 5oC, increases to 6.7 at 25oC. When the temperature drops to 5oC, the pH returns to 6.5.
Another unit used to express milk acidity is the Soxhlet-Henkel (oSH). The SH degree of milk is determined by the titration method (How milk acidity is determined by titration is explained below).
The acidity level of milk freshly milked from a healthy animal is between 6.4 and 7.0 oSH. As acidity increases, the SH degree of milk also increases. In general, 8.0 oSH means acidity has begun; therefore, milk with acidity above this value is generally considered unsuitable for processing. When the acidity increases to 10.0 oSH, clot formation usually occurs when milk is heated. At 25-30 oSH values, milk may show coagulation when at room temperature. The SH value of milk with mastitis is generally between 5.0 and 4.0.
However, it should be noted that while pH measures the amount of free hydrogen ions in the environment, SH measures the total amount of acid in the environment. The two are quite different parameters. This difference is quite significant in some cases. As a matter of fact, sometimes, due to differences in breeding or animal ration, the milk may not coagulate due to the effect of heat, even if the acidity of the milk is 10-12 oSH. On the other hand, as the rate of the titratable serum phase decreases, the titration method becomes inconsistent. Therefore, measuring pH value instead of SH would be more consistent, especially in dairy industry.
The % acidity value of milk in terms of lactic acid (milk acid) (abbreviated as % acidity) is important in that it is accepted as a criterion for acidity by the Ministry of Agriculture and Forestry (Türkiye) and is a criterion used in the literature. At the same time, this method is the most commonly used method in America.
As a matter of fact, the Ministry of Agriculture and Forestry (Türkiye) determines the minimum and maximum amount of acid that should be in raw milk and dairy products according to the % acidity value in terms of lactic acid (milk acid).
The acidity of cow’s milk freshly milked from a healthy animal is between 0.14% and 0.16%. Adding water to milk causes a decrease in milk acidity of 0.002%.
The Ministry of Agriculture and Forestry (Türkiye), in its Communiqué on Raw Milk Supply (2017/20), stated that the minimum and maximum acidity values that milk of different animals should have are as follows;
• Cow’s milk; 0.135 – 0.200%
• Sheep milk; 0.160 – 0.350%
• Goat milk 0.150 – 0.280%
• Buffalo milk; 0.140 – 0.220%.
The Ministry of Agriculture and Forestry (Türkiye) states in the Turkish Food Codex Fermented Dairy Products Communiqué (2022/44) that the minimum and maximum acidity that dairy products should have should be as follows;
• Fermented milk products; at least 0.3%,
• Yogurt; minimum 0.6%, maximum 1.5%,
• Ayran; at least 0.5%, at most 1%,
• Kefir; at least 0.6%,
• Kumiss; at least 0.7%,
• Strained yogurt; minimum 0.6%, maximum 2.0%,
• Concentrated fermented milk product;at least 0.6%, at most 1.5%.
Dornic (oD) and Thörner (oT) are other grades used for milk acidity by titration method.
Essentially, the only difference in determining Soxhlet-Henkel (oSH), % acidity, Dornic (oD) and Thörner (oT) degrees is the difference concentration of the NaOH solution used in the titration.
In this respect, each of these four degrees can be converted to another mathematically. The % acidity can be converted to SH degree by dividing by 0.0225. Likewise, the % acidity value in terms of lactic acid (milk acid) can be found by multiplying the SH degree by 0.0225.
For example, the acidity value of milk, whose acidity is 6.4 oSH, can be found as 0.144 % acidity by multiplying it by 0.0225. (6.4 x 0.0224 = 0.144)
The SH degree of milk with a % acidity of 0.160% is 7.11 oSH. (0.160/0.0225 = 7.11)
The equivalence of milk acidity units determined by the titration method is given in the table below;

While it is possible to convert these four units to each other mathematically, pH has no connection with these values, and therefore, pH cannot be mathematically converted to any of these values.
Methods to Determine Milk Acidity
Many different methods can be used to determine milk acidity. While the degree of acidity can be determined in some of these methods, in others, it can only be determined observationally whether the acidity is high or not.
Methods that allow the degree of acidity to be determined are called “objective methods”, while methods that cannot determine the amount of acidity but only provide an idea that the acidity has increased are called “subjective methods.” Methods for determining acidity in milk are given in the diagram below;
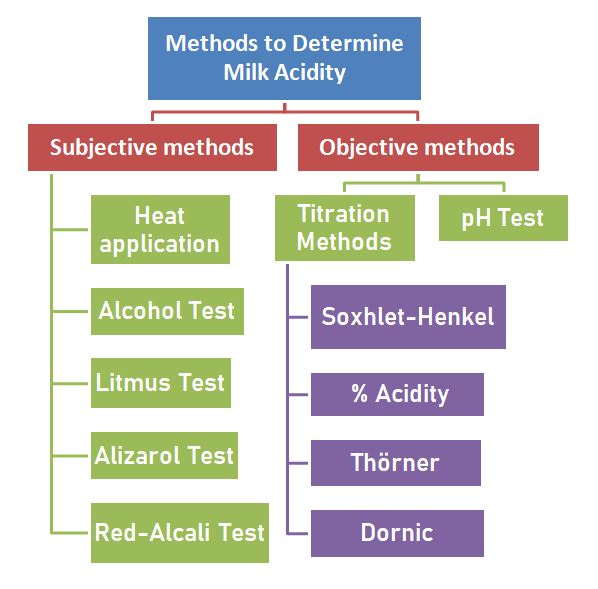
However, some of these methods have lost their use today. Methods frequently used in the food industry and academia are pH test, Soxhlet-Henkel, % acidity, alcohol test and heat application.
1. Determination of milk acidity with a pH meter
Determining milk acidity via pH is quite simple and practical. Thanks to devices called “pH meters”, the pH of milk can be measured instantly. For this purpose, the probe of the pH meter is immersed in milk and instant measurement is performed. Before measurement, the milk must be mixed and the milk temperature must be around 20oC.

The pH meter should be calibrated routinely and periodically according to the frequency of use. Calibration of the pH meter is a must for accurate results.
The tip of the probe should not touch the surface of the milk container; it should only be immersed in contact with milk. Otherwise, the measurement may be incorrect. On the other hand, the probe tip is very sensitive, and sudden contact with a hard surface may cause the probe tip to break. The stylus is a replaceable part and a bit expensive. It is necessary to be very careful when using it; it should be rinsed with pure water and dried before and after use. When not in use, the probe should be kept submerged in the preservative solution (preservative solution is usually potassium chloride solution). On the other hand, the pH meter probe inevitably loses its ability over time and becomes unable to measure. Proper use and storage extend the life of the pH meter probe.
When purchasing a pH meter, it is necessary to purchase the buffer solutions used in calibration.
2. Determination of milk acidity by the Soxhlet-Henkel method
It is a titration method. In the titration, 0.25 N NaOH solution is used as a neutralizer and 2% phenolphthalein is used as a color indicator.
Before taking samples, the milk should be at 20oC and mixed thoroughly. 25 mL of milk is placed in a 100 mL conical flask, 1 mL of 2% phenolphthalein solution is added and 0.25 N NaOH solution is added drop by drop through a burette or pipette. The flask is constantly shaken. The titration is terminated when the white milk color turns permanent light pink.
The SH degree of the milk is determined by multiplying the amount of sodium hydroxide solution spent in the titration by 4.
For example, if 1.7 mL of 0.25 N NaOH solution was used in the titration, the SH degree of the milk;
1.7 x 4 = 6.8 oSH.
oSH = NaOH solutions required for neutralization (mL) x 4
In general, the point to be considered in titration analyses is that the analyses must be carried out by a single person. Even though they are experts, different people’s hand sensitivities may differ and therefore slightly different results may be obtained.
3. Determination of milk acidity by the % acidity method
It is a titration method. 0.1 N NaOH solution is used as a neutralizer and 2% phenolphthalein solution is used as a color indicator.
Before taking samples, the milk should be at 20oC and mixed thoroughly. Weigh 18 grams of milk on a sensitive scale in a 100 mL conical flask and add 1 mL of 2% phenolphthalein solution. 0.1 N NaOH solution is added dropwise. The flask is shaken continuously and the titration is terminated when a permanent light pink color is observed. The % acidity value of milk is calculated by substituting the values in the formula below;

The reason for using an 18-gram sample in this analysis is that it provides simplification in the formula. As a result of this simplification, the amount of NaOH spent in titration is directly multiplied by 0.05 to find the % acidity value.
4. Determination of milk acidity by alcohol test
The alcohol test is a subjective test and does not provide precise information about the degree of acidity; it is a method that only provides an idea about whether the acidity is high or not. It is a frequently used method by dairy farms to purchase milk from farmers.
If pasteurization is to be applied to the milk purchased, equal amounts (usually 5% mL each) of 68% ethyl alcohol are mixed with the milk and observed whether a clot forms. If clot formation is observed, it is accepted that the acidity of the milk has increased, and therefore, it cannot withstand the heat treatment applied during pasteurization.
If the milk to be taken will be subjected to high heat treatment such as the UHT process, equal amounts of 72% or 74% ethyl alcohol are mixed with the milk sample and observed whether a clot forms. If 68% ethyl alcohol is used to test resistance to the UHT process, two units (10 mL) of 68% ethyl alcohol are mixed with 1 unit (5 mL) of milk sample.
If there is no clot formation, it is concluded that the milk can with stand the heat treatment to be applied. However, any form of clot formation means that the milk cannot withstand the heat treatment and will curdle.
Clot formation in the alcohol test can be interpreted regarding SH value. No clots mean the acidity is lower than 8 oSH. Very small point-shaped clot formation 8-8.5 oSH; tiny clot fibers 8.5-9 oSH and the appearance of large clot pieces corresponds to values of 9-10 oSH.
Another benefit of the alcohol test is that even if the acidity is at normal levels, colostrum and milks with advanced mastitis also coagulate under the influence of alcohol, thus preventing the acceptance of these kinds of milk.
5. Determination of milk acidity by heat application
It is the most practical and cheap method for determining milk acidity. It can be applied with just a teaspoon and a lighter, or it can be done at home by testing a tablespoon of milk on the stove.
To determine the acidity, the milk placed in a spoon is allowed to boil and clot formation is observed. If there is clot formation, it is concluded that the acidity of the milk has increased and, therefore, it cannot withstand heat treatment. It is a method that can be used practically at home. In this way, whether the milk is consumable or not can be easily understood.